Agostina Casamento-Moran, Post Doctoral Studies in Computational Neuroscience


Agostina Casamento-Moran, a post-doctoral fellow at Johns Hopkins University, is one of the winners of the 2023 Seed for Science initiative for her research project Physiological Mechanisms of Post-Exertional Malaise in Long COVID. To support further research of her project, the De Luca Foundation has awarded her a $10,000 stipend, EMG equipment from Delsys, and other biofeedback sensors from Moxy, VO2 Master, and Polar.
Long COVID is an uncommon condition that can lead to permanent physiological changes in patients after they have contracted COVID-19. There are very few studies exploring the mechanisms of Long COVID, but the impact of the condition continues to grow:
19% of people who contract COVID-19 will develop Long COVID1
Post-exertional malaise, or PEM, is the most common symptom for individuals with Long COVID
It is predicted that Long COVID will exacerbate the $20 billion annual economic burden of PEM1
Casamento-Moran believes that the cause of PEM in Long COVID can be traced to cardiovascular dysregulation and impaired oxygen extraction, as seen in a similar condition, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)5. Using the pre-existing research into ME/CFS and the resources provided by the De Luca Foundation, Casamento-Moran will gather the data she needs to help expand our understanding of Long COVID:
Trigno EMG: Measuring the rate of neuromuscular activations
Moxy: Comparing muscle oxygenation to perceived fatigue
VO2 Master: Measuring the efficiency of exercise ventilation
Polar: Monitoring heart rate variability during various activities
“People with long covid or chronic fatigue syndrome have this phenomenon called post-exertional malaise, which is that after they exert themselves, even if it’s activities of daily living or submaximal stuff, they will fall in this crash of energy where some of them become bedbound. And why that’s happening and what’s triggering that and how we can behaviorally characterize that post-exertional malaise, we don’t have a clue.”
Agostina Casamento-Moran
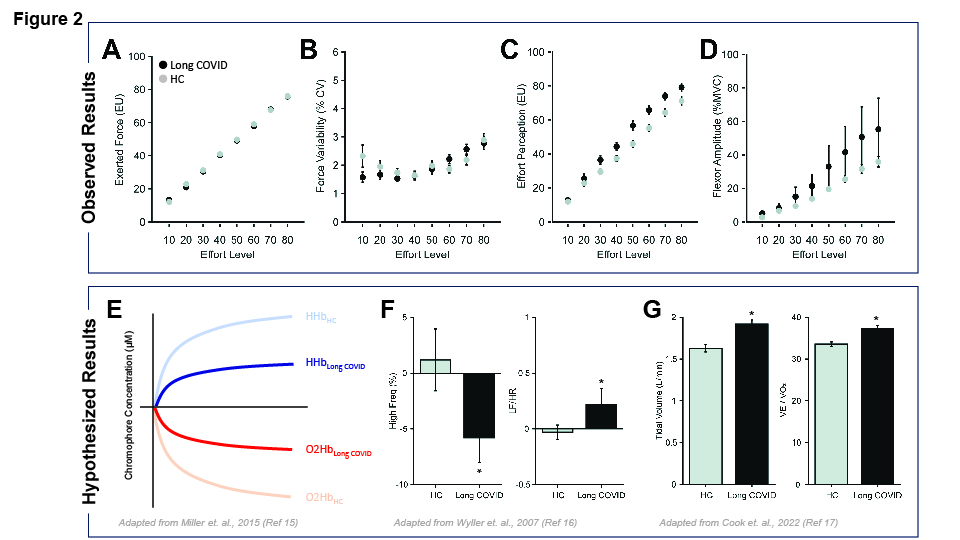
Casamento-Moran’s preliminary research has found that individuals with long COVID demonstrate increased neuromuscular activation and perceived levels of effort during exercise compared to a healthy control group (fig. 2A, B, 2C, 2D). In the current study, she plans to have her participants go through a series of tests and surveys to reveal how PEM can affect peoples’ everyday lives. Casamento-Moran hopes to shed light on the mechanisms of Long COVID and how they can lead to PEM.
References
- “Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness” (2015), , doi:10.7205/MILMED-D-15-00085.
- M. Van Herck et al., J Med Internet Res. 23 (2021), doi:10.2196/30274.
- Z. Al-Aly, Y. Xie, B. Bowe, Nature. 594, 259–264 (2021).
- D. M. Raizen et al., Sleep (2023), doi:10.1093/sleep/zsad069.
- A. L. Komaroff, W. I. Lipkin, ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med (Lausanne). 10 (2023), , doi:10.3389/fmed.2023.1187163.
- P. Joseph et al., in Chest (Elsevier Inc., 2021), vol. 160, pp. 642–651.
- I. Singh et al., Chest. 161, 54–63 (2022).
- P. Joseph et al., Exercise Pathophysiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Postacute Sequelae of SARS-CoV-2: More in Common Than Not? Chest. 164 (2023), pp. 717–726.
- L. Chu, I. J. Valencia, D. W. Garvert, J. G. Montoya, Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS One. 13 (2018), p. e0197811.
- T. E. Davenport, S. R. Stevens, K. Baroni, M. Van Ness, C. R. Snell, Disabil Rehabil. 33, 1768–1775 (2011).
- K. Sommerfelt, T. Schei, K. A. Seton, S. R. Carding, (2023), doi:10.20944/preprints202309.2091.v1.
- D. U. Jette et al., Phys Ther. 94, 379–391 (2014).
- S. Perrot, M. Lantéri-Minet, European Journal of Pain (United Kingdom). 23, 1117–1128 (2019).
- P. S. Hogan et al., Nat Commun. 11 (2020), doi:10.1038/s41467-020-17855-5.
- R. R. Miller et al., J Transl Med. 13 (2015), doi:10.1186/s12967-015-0527-8.
- V. B. Wyller, J. P. Saul, L. Walløe, E. Thaulow, Eur J Appl Physiol. 102, 623–632 (2008).
- D. B. Cook et al., PLoS One. 17 (2022), doi:10.1371/journal.pone.0265315.

